Subtotal: $NZ 19.99
Australian Nicotine Prescriptions & Import Laws
This means Australian customers will no longer be able to import ANY vape products purchased directly from overseas suppliers like Vapeys, even if they have a nicotine prescription. This is a serious change that will have a negative impact on all of our Australian customers.
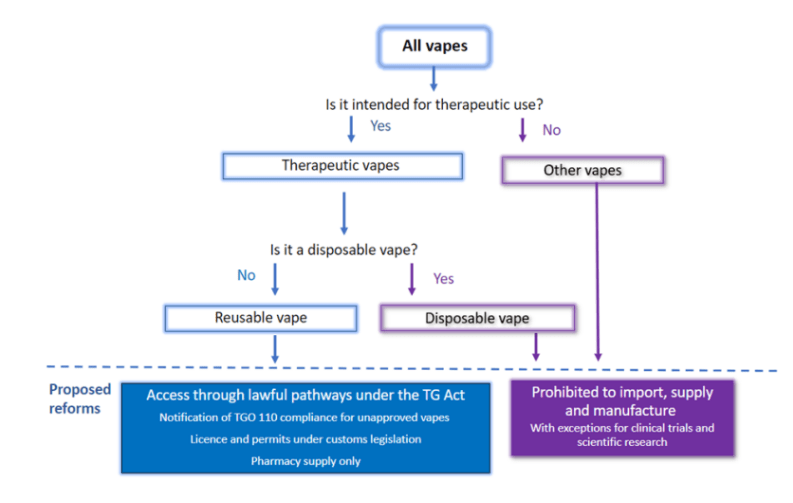
The importation of vaping goods is subject to regulation 5A of the Customs (Prohibited Imports) Regulations1956-(PI Regulations).
From 1 January 2024, the import of disposable vapes is prohibited unless the importer holds a licence and permit issued by the Office of Drug Control (ODC) under the PI Regulations.
From 1 March 2024, the import of other vaping goods, including devices, accessories, and substances, will require a licence and permit issued by the ODC.
Only businesses will be eligible to obtain import licences and permits for vaping goods.
Your company or business will need to meet certain criteria to be granted a licence or a permit to import vaping goods into Australia. A permit to import each specific type of vaping good is required.
Examples of vaping devices (not a complete list):
Vapes (reusable)
Vapes (disposable)
E-cigarettes
E-cigars
Dry herb vaping devices
510 batteries
510 battery powered vapes
A vape is a device (whether or not filled with a vape substance) that generates or releases using a heating element and by electronic means, an aerosol, vapour or mist for direct inhalation by its user. This includes parts of the vape including batteries (e.g. 510 batteries) that provide the electronic mechanism to heat the element to produce the aerosol, vapour or mist.
Examples of devices that are not vapes include the following:
humidifiers
diffusers
nebulisers
inhalers.
Before an import permit can be granted under a vaping good import licence, the vaping good must either be included in the Australian Register of Therapeutic Goods (ARTG), or a notice must be given to the Therapeutic Goods Administration (TGA) which complies with applicable requirements under the Therapeutic Goods Act 1989.
For more information on the notification process and why these changes are taking place, visit the Vaping hub | Therapeutic Goods Administration (TGA)- external site, or contact the Therapeutic Goods Administration (TGA).
Additional detail on the vaping goods covered by the PI regulations is available in Australian Customs Notice No. 2023/51- external site. You can also contact [email protected] if you require further advice regarding the import of vaping goods.
Further information on the regulation of vaping goods can be found on the Therapeutic Goods Administration Vaping Hub- external site.

 Solo Plus-2800 Puffs- 20mg/ml Nicotine - Disposable Vape - SOUR RASPBERRY, 2%
Solo Plus-2800 Puffs- 20mg/ml Nicotine - Disposable Vape - SOUR RASPBERRY, 2% 